STUDY
This study aims to gain more insight in the immunological characteristics of SARS-CoV2 infection. Samples have been collected from patients with a high clinical suspicion of COVID-19, hospitalized at either UZ Gent, AZ Maria Middelares Gent or AZ Jan Palfijn Gent, Belgium.
SAMPLE TYPES
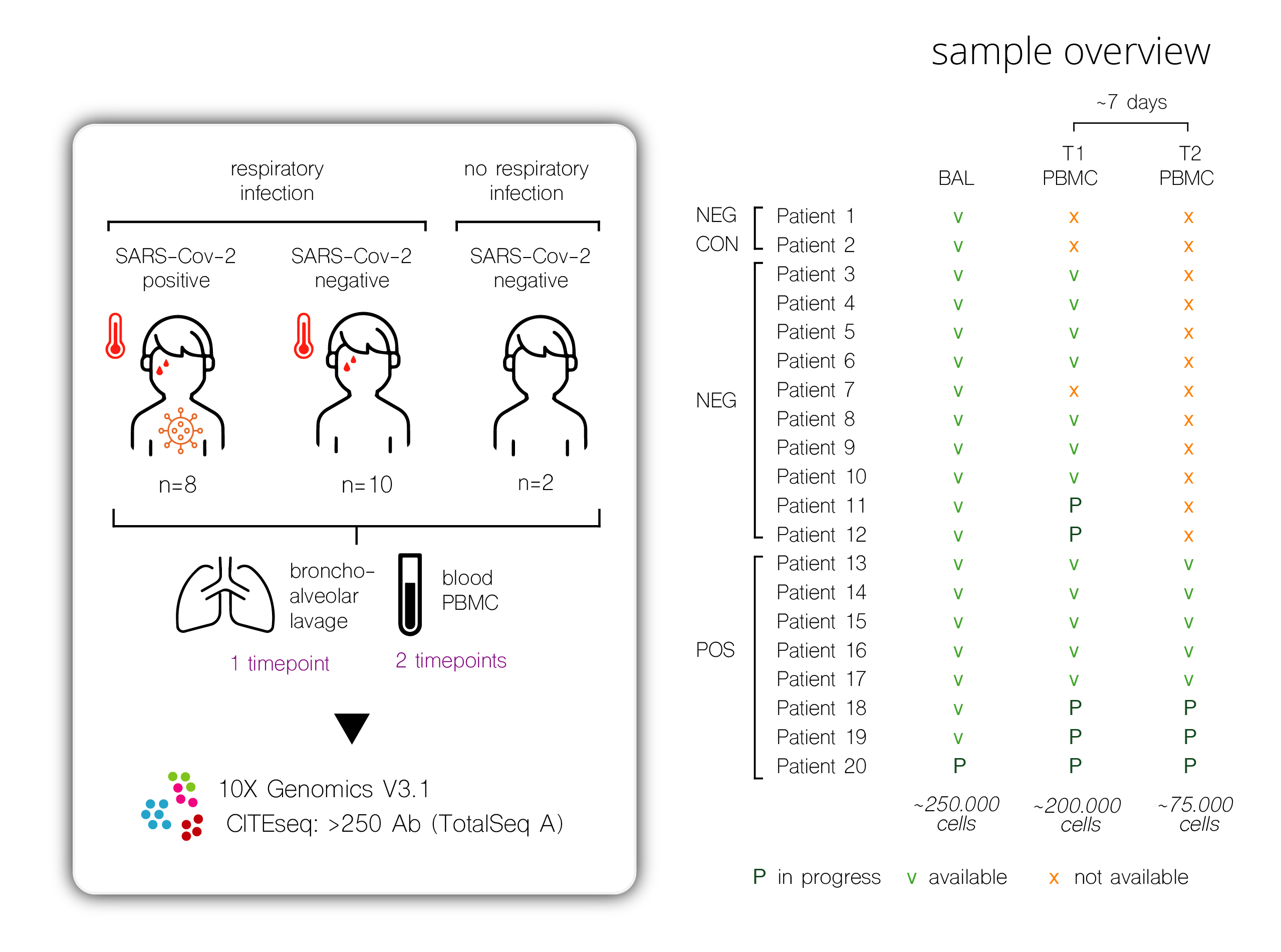
In patients with a diagnostic or therapeutic need for bronchoscopy, we sampled bronchoalveolar lavage fluid (BALF), nasopharyngeal swabs, serum, plasma and peripheral blood mononuclear cells (PBMC). Patients were included in clinical trials under supervision of prof. dr. Bart Lambrecht and prof. dr. Linos Vandekerckhove.
IMMUNOLOGICAL ASSAYS
Applications include single-cell RNA-sequencing along with the quantitative measurement of surface proteins using panels of 275 oligo-conjugated antibodies (CITEseq); scATACseq, multiparameter flow cytometry, Meso Scale biomarker assays; antibody/neutralization assays.
EARLY DATA ACCESS
CITEseq/single-cell transcriptome datasets generated through this project within the VIB/Ugent single cell platform ‘Singularity’ are made publicly available in the early stages of the project. This will allow the wider research community to rapidly explore the data, which for example could be used for drug repurposing studies and reveal combination therapies of existing drugs that are most likely to succeed in the clinic. All single-cell datasets are readily contributed to the Covid-19 Cell Atlas.
principal investigators
contact
partners